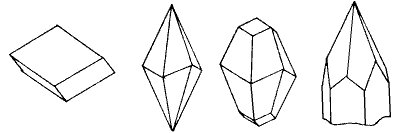
Fig. 1: common shapes of calcite crystals drawn with c-axis vertical
| rhombohedron | scalenohedron | rhomb + scalenohedron | scalenohedron + prism |
This article attempts to deal with the comoner mineral formations deposited in British caves, and to look at the principles that govern their origins.
Few minerals are present in British caves as compared with caves of warmer climates, a consequence of the fact that most British caves are situated in pure limestone. In warmer areas, such as the Mississippi region of America, many caves are situated in Dolomitic limestones where a range of magnesium minerals is found (2). By far the most common mineral is calcite, followed by gypsum, iron and manganese oxides, and possibly aragonite. A greater variety of secondary minerals is found in mines, because of the availability of heavy metal ions from the ore veins
Calcite and aragonite are both calcium carbonate, CaCO3, in different crystal forms. Calcite is trigonal, and stable under normal conditions, while aragonite is orthorhombic, and stable at pressures above 5000 atmospheres. Calcite is much more common than aragonite, although aragonite can crystallize metastably in the presence of Magnesium. The presence of aragonite in British caves seems doubtful, though it has been reported in Lamb Leer. Calcite and aragonite are difficult to distinguish without laboratory equipment.
Common forms of calcite crystal are shown in fig. 1. These are developed in crystal pools. An important property of calcite is its rhombohedral cleavage: it invariably breaks parallel to the faces of a rhombohedon, even where these these are not present on the surface, and hence the c-axis of the crystal structure can easily be located.

| rhombohedron | scalenohedron | rhomb + scalenohedron | scalenohedron + prism |
Gypsum is a hydrated calcium sulphate, CaSO4.2H2O. It forms good platy crystals, or flat prisms, and is readily distinguished from calcite. The crystals are often found growing in dry mud deposits. Selenite is an alternative name for well crystallized gypsum.
Various hydrated iron and manganese oxides are occasionally found in caves, and more frequently in mines. They are usually soft orange to black masses without crystal form. Ochre and "limonite" come within this category.
As the cavernous limestones of Britain contain more than 90% calcite a knowledge of the chemical relationship between calcite, water and atmospheric carbon dioxide is essential to the understanding of cave formation and calcite speleothems. The process that leads to speleothem formation is essentially the opposite of the process of cave formation.
Calcite is slightly soluble in water, the amount depending on the amount of carbon dioxide in the water, which depends on the amount present in the atmosphere. This is governed by the equilibria
CaCO3 <=> Ca2+ + CO32-
CO32- + H+ <=> HCO3-
HCO3- + H+ <=> H2CO3
H2CO3 <=> H2O + CO2
H2O <=> H+ + OH-
Water passing through the soil zone, whose atmosphere may contain up to 3% carbon dioxide, is able to dissolve calcite, enlarging fissures in the limestone, reaching concentrations of up to 200ppm. The atmosphere in a cave contains approximately .04% carbon dioxide. Water in equilibrium with this atmosphere can contain only 20ppm of calcite. The excess calcite may be precipitated as speleothems given suitable conditions, as the water reequilibriates. Loss of carbon dioxide is the most important factor in calcite precipitation, as evaporation in near saturated cave atmospheres is very low.
The source of sulphate for gypsum formations is less clear. In S. Wales, it may be derived from iron sulphides in the black limestones by oxidation
2FeS2 + 2H2O + 7O2 =>
2FeSO4 + 2H2SO4
CaCO3 + H2SO4 =>
CaSO4 + H2O + CO2
Such a reaction does not readily explain the gypsum in the white limestones of Yorkshire. Gypsum is soluble in water, and is precipitated only when the water evaporates. It tends to be limited to old dry passages where such evaporation is possible, though it is present at the head of Nether Rawl, Cwm Dwr, in a stream passage.
Hydrated iron oxides are the end result of the oxidation above :-
FeSO4 + 5H2O + ½02 => 2Fe(OH)3 + 2H2SO4
The actual reaction may be caused by chemolithotropic bacteria. These formations tend to form where percolation water enters larger passages, and oxygen becomes available
Most calcite formations fit into two categories, those where the shape is governed by a film of water, and those where the shape is governed by the calcite crystals themselves. The former category includes the bulk of stalactite, stalagmites and flowstones, and will be considered first.
In the high humidity of most cave atmospheres the surface tension of water ensures that a continuous film of solution covers the surface of calcite formations, but not the edges. As the crystals must remain in the film to grow, the crystal form is suppressed and the usual bulbous shapes of speleothems are produced. If the water flows at a measurable rate, the viscosity of the water causes the development of a thick film, with the formation of a visibly crystalline surface, still governed by the general shape of the water film. The tendency of the water film to cover smooth surfaces means that there is little damping should an irregularity develop, which enables a knobbly surface to form. At high flow rates, the water flow becomes turbulent, and a series of ripples begin to form in the calcite surface, frequently noticeable on gour dams.
Nucleation phenomena affect all calcite formations. It is much easier to add to an existing crystal than to start a new one provided that the surface is clean. Thus calcite formations contain relatively few crystals of great length. The likelihood of fresh nucleation is increased by a large degree of supersaturation; in Minera mine, where the original calcite concentration is around 250ppm, in Ogof Fynnon Ddu, formations contain many more crystals.
Not all faces of a crystal grow at the same rate. The fastest growing faces of a calcite crystal are usually those normal to the c-axis, leading to crystals elongated parallel to the c-axis. Competitive growth fabrics, illustrated in fig 2, are the result of this.
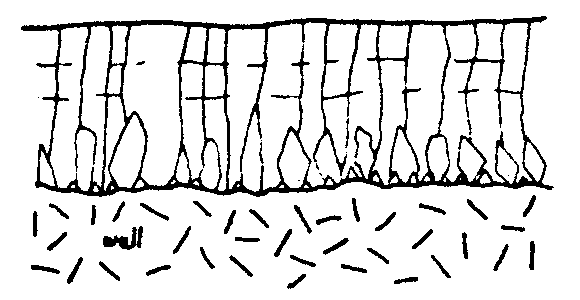
Broughton, 1977, has demonstrated that the growth of calcite crystals is probably a two stage process, starting with an aggregate of near parallel crystallites, later filled in to produce a "single crystal". Detailed examination of such a crystal reveals its origin. This origin explains the difficulty with which good competitive growth fabrics are observed. Throughout the rest of this article the term crystal will refer to such individuals, which usually show a continuous cleavage when observed by the naked eye.
Fig. 3 Initial stage of straw formation
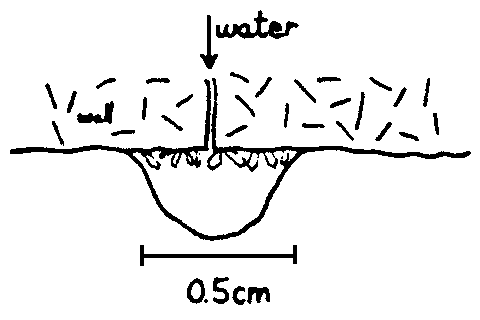 We can consider the growth of a straw stalactite, the
simplest kind, from the first drop. A slow supply of calcite laden water
drips from a hole in the roof. As each drop hangs it loses some carbon
dioxide, and has a tendency to deposit calcite. Many randomly orientated
crystals will be produced within the drip volume (fig 3). As further
precipitation continues, competitive growth will eliminate all but a few
"crystals" growing with the c-axis vertical (fig 4).
We can consider the growth of a straw stalactite, the
simplest kind, from the first drop. A slow supply of calcite laden water
drips from a hole in the roof. As each drop hangs it loses some carbon
dioxide, and has a tendency to deposit calcite. Many randomly orientated
crystals will be produced within the drip volume (fig 3). As further
precipitation continues, competitive growth will eliminate all but a few
"crystals" growing with the c-axis vertical (fig 4).
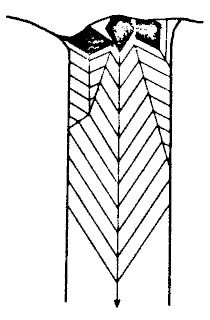 | 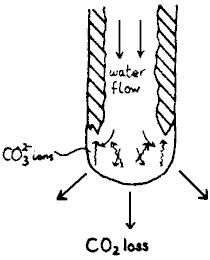 |
| Fig. 4 Diagrammatic illustration of competitive growth in a straw |
Fig. 5 Changes at end of straw |
The hollow shape is produced by the flow pattern at the end of the straw (fig 5). The flow prevents CO ions formed at the drop surface from diffusing back to the precipitation site, except at the turbulent edges. If the flow stops, there is a chance that precipitation will close the tube; conversely if the flow is too rapid, very little calcite will be precipitated, thus straw growth only takes place within a narrow range underground. Growth rates of around 1 mm per year seem to be the norm (Moore 1962).
Fig. 6 Diagrammatic cross-section of stalactite.
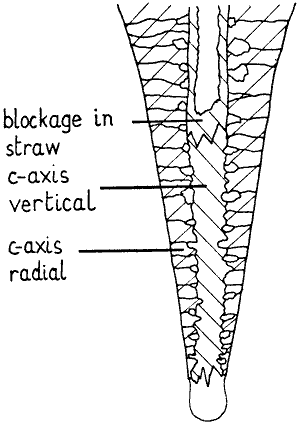 The
more normal carrot shaped stalactite may develop from a straw, or may never
be hollow. Many stalactites can be seen to consist of a central tube of
"crystals" with c-axis vertical, surrounded by "crystals" with c-axis
radiating, representing two phases of growth. In later stages, the situation
is as represented in fig 6. The arrangement of "crystals" is such that their
fastest growing faces are presented to the solution. (It has been suggested
that for some reason the flow inside a straw may switch to the outside,
perhaps because of a blockage of the straw. However it is difficult to see
how any appreciable flow could get from the inside to the outside of an
essentially impermeable staw, a composite source of water seems more
probable, with the two crystal orientations occurring simultaneously). Much
rarer are stalactites consisting of a single vertically oriented "crystal".
These are formed at flow rates too low for straws. It is interesting to note
that icicles, which do not form straws, are of this type.
The
more normal carrot shaped stalactite may develop from a straw, or may never
be hollow. Many stalactites can be seen to consist of a central tube of
"crystals" with c-axis vertical, surrounded by "crystals" with c-axis
radiating, representing two phases of growth. In later stages, the situation
is as represented in fig 6. The arrangement of "crystals" is such that their
fastest growing faces are presented to the solution. (It has been suggested
that for some reason the flow inside a straw may switch to the outside,
perhaps because of a blockage of the straw. However it is difficult to see
how any appreciable flow could get from the inside to the outside of an
essentially impermeable staw, a composite source of water seems more
probable, with the two crystal orientations occurring simultaneously). Much
rarer are stalactites consisting of a single vertically oriented "crystal".
These are formed at flow rates too low for straws. It is interesting to note
that icicles, which do not form straws, are of this type.
The rate of calcite precipitation is a function of the amount of calcite in the solution, a fact that produces the tapering shape of stalactites. Most calcite is deposited at the top of the stalactite, reducing the rate of deposition further down and so on. If simplifying assumptions are made, mathematical equations for the shape of stalactites can be made:-
| thickness of water film (constant) | = | |
| flow rate | = | |
| density of calcite | = | |
| initial concentration of calcite | = | |
| final concentration of calcite | = | 0 |
| initial radius of straw | = | r0 |
| deposition rate of calcite governed by dm/dt | = | k |
where A is the area, then the radius r at distance z from the top at time t will be given by
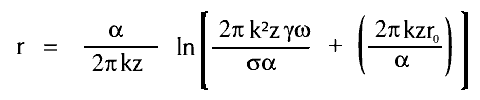
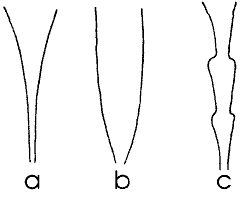
Fig. 7 Shapes of stalactites
 The solution of more realistic models of stalactite growth may be possible
with the aid of computers, but little progress has been made in this field.
To produce shapes other than the simple carrot (with concave faces), fig. 7a,
further considerations are necessary. Flow rate, calcite content and degree
of saturation are variable on a seasonal scale and a long term scale, and may
account for convex carrots. Such changes could be treated by computer models.
As straws are frequently porous, when they block, stalactite growth may begin
from several places, fig. 7c. These undulations will become less significant
as the stalactite grows. The irregularities in large stalactites may become
such that secondary forms such as curtains develop.
The solution of more realistic models of stalactite growth may be possible
with the aid of computers, but little progress has been made in this field.
To produce shapes other than the simple carrot (with concave faces), fig. 7a,
further considerations are necessary. Flow rate, calcite content and degree
of saturation are variable on a seasonal scale and a long term scale, and may
account for convex carrots. Such changes could be treated by computer models.
As straws are frequently porous, when they block, stalactite growth may begin
from several places, fig. 7c. These undulations will become less significant
as the stalactite grows. The irregularities in large stalactites may become
such that secondary forms such as curtains develop.
The growth of stalagmites is at first sight similar to that of stalactites
- certainly the depositional mechanism and variables present are identical.
Stalagmites differ in two ways, they grow against gravity rather than with
it, and the starting shape is usually a horizontal plane, not a vertical
cylinder. Stalagmites result from water falling on the floor in a well
defined place from which a sheet of water spreads out. Mathematical treatment
of such a situation is not satisfactory, as the effect of gravity in the
centre cannot be treated. Fig. 8. 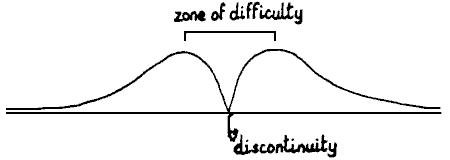 In a real stalagmite, this zone of
difficulty is removed by the splashing that takes place on drop impact. The
result of these processes is that a blunt cone grows from the centre of the
sheet. The two variables, flow rate and available calcite, control the shape;
low flow and high calcite produces a narrow cone, almost cylindrical e.g. the
Columns OFD; high flow produces more rounded forms e.g. the Hall of the
Thirty, Otter Hole. By assuming an equilibrium shape, Franke 1961, 1962, has
had some success in calculating growth.
In a real stalagmite, this zone of
difficulty is removed by the splashing that takes place on drop impact. The
result of these processes is that a blunt cone grows from the centre of the
sheet. The two variables, flow rate and available calcite, control the shape;
low flow and high calcite produces a narrow cone, almost cylindrical e.g. the
Columns OFD; high flow produces more rounded forms e.g. the Hall of the
Thirty, Otter Hole. By assuming an equilibrium shape, Franke 1961, 1962, has
had some success in calculating growth.
Fig. 9
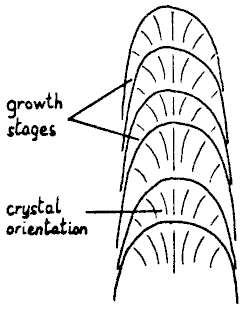 Crystal orientation in stalagmites is more variable than in stalactites,
though governed by the same principles. As a consequence of the processes
described above, a stalagmite can be considered as a series of superimposed
conical shapes, fig. 9. The desirability of the crystals growing c-axis
normal to the surface produces a mushroom pattern, best developed when
conditions allow frequent renucleation.
Crystal orientation in stalagmites is more variable than in stalactites,
though governed by the same principles. As a consequence of the processes
described above, a stalagmite can be considered as a series of superimposed
conical shapes, fig. 9. The desirability of the crystals growing c-axis
normal to the surface produces a mushroom pattern, best developed when
conditions allow frequent renucleation.
Fig. 10 Diagrammatic illustration of curtain
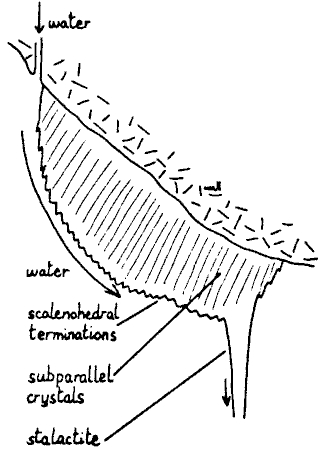 Curtains are akin to stalactites in growing on the ceiling, and akin to
stalagmites in involving running, not dripping water. A sloping roof is
essential, hence their rarity in the horizontal limestones of Yorkshire.
Water running down the slope follows a narrow tortuous track, and leaves a
thin ribbon of calcite. Competitive growth soon turns this into an array of
sub-parallel 'crystals' growing away from the wall. The stream of water
follows the outermost edge only, leading to a sheet 5mm thick, fig. 10. If
the curtain does not rejoin the wall, dripping at a point takes place, and a
stalactite grows from the curtain. The forms with which we have been dealing
have been governed by water under the influence of gravity. Forms determined
by calcite crystallization are further divisible into those in which water is
present in a film, which we will now consider, and those in which it is
present as a thick layer.
Curtains are akin to stalactites in growing on the ceiling, and akin to
stalagmites in involving running, not dripping water. A sloping roof is
essential, hence their rarity in the horizontal limestones of Yorkshire.
Water running down the slope follows a narrow tortuous track, and leaves a
thin ribbon of calcite. Competitive growth soon turns this into an array of
sub-parallel 'crystals' growing away from the wall. The stream of water
follows the outermost edge only, leading to a sheet 5mm thick, fig. 10. If
the curtain does not rejoin the wall, dripping at a point takes place, and a
stalactite grows from the curtain. The forms with which we have been dealing
have been governed by water under the influence of gravity. Forms determined
by calcite crystallization are further divisible into those in which water is
present in a film, which we will now consider, and those in which it is
present as a thick layer.
Included in this category are speleothems variously referred to as
helictites, heligmites, anemolites, erratics etc. Many different explanations
have been proposed for these forms, and probably most of them are valid in
particular cases (see Huff, 1940). One of the most frequent suggestions is
that of wind deflecting drops of water. Such a mechanism may be the cause of
gently curved stalactites in obviously draughty places, and these may
justifiably be called anemolites. Interesting eccentricities are to be found
in Nant Newydd, Ogof Ffynnon Ddu. 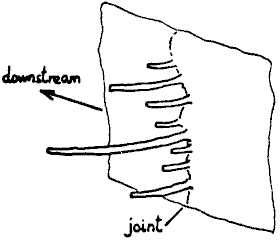 These grow
horizontally from cracks in a vertical limestone wall, and curve downstream,
fig. 11. The draught may produce the curvature, but it is unlikely to be
responsible for the horizontal growth.
These grow
horizontally from cracks in a vertical limestone wall, and curve downstream,
fig. 11. The draught may produce the curvature, but it is unlikely to be
responsible for the horizontal growth.
The random change in shape of many small eccentrics is probably in most cases a result of irregular crystal growth. Detailed petrographic studies are needed to confirm this (see Huff, 1940). The other vital factor is the absence of dripping water, which would impose its own shape on the eccentric. Capillary rise may be important to supply water uphill to eccentrics, often aided by hydrostatic pressure. More problematical by far is how to remove the constant supply of water without dripping or evaporation taking place.
Eccentrics may develop from hollow stalactites or from solid sheets of flowstone. Development from a straw is probably a consequence of the straw choking because of low flow, continued low flow favouring the formation of an eccentric rather than a conical stalactite. The reason for growth from flowstone is obscure. Eccentrics in both situations may be solid or hollow, the hollow ones frequently having a much smaller diameter than a straw.
Further complications are caused by eccentrics such as the Fingers, OFD I, which have blunt rounded shapes and are white rather than translucent. It can be seen that eccentrics cover a wide field, with many different problems.
In cave pools are found those speleothems whose form is governed by calcite crystals unrestricted by water films. Such speleothems are crystals and cave pearls.
In a static pool crystals are free to develop their normal shapes, scalenohedra and rhombohedra being the most common The shape formed depends in a little known fashion on the degree of supersaturation, and the trace element composition of the water. The size and regularity of the crystals increases as the rate of deposition decreases, provided the pool is undisturbed and free from insoluble matter. Frequently deposition is rapid, and a sharp prickly mass is formed, such as coats much of Ingleborough Cave, rather than the recognisable crystals.
If much agitation is present, so that abrasion of deposited calcite can occur, cave pearls are formed. These are shiny concretions consisting of fine radially orientated calcite coating nuclei. The concretions are often found in large numbers filling pools to the extent that they may eventually force each other out (Baker & Frostick, 1947). Falling water is the source of agitation, though pearls grow to such a size that this has been debated.
Some of the calcite deposited from cave waters is the result of bacterial activity. The most obvious example of this is the deposit moonmilk, such as found abundantly in OFD. However many other formations, such as the cauliflower-like material in Disappointment Pot may be partly organic in origin.
The deposits in S. Wales have been shown to be associated with the bacterium Macromonas bipunctata, (Mason-Williams, 1961). The bacterium metabolises organic acids, and calcite is precipitated in the unusual form lublinite, though the details of this process have not yet been resolved. Lublinite is an unstable form, and tends to recrystallize, causing solidification of the mass. Studies of this phenomenon are in progress. Elsewhere bacteria, and also fungi and blue-green algae have been shown to be associated with calcite and magnesium mineral precipitation.
Compared with the diversity of calcite formations, gypsum formations are extremely uniform. All are variations on the theme of crystals, either in mud or on other speleothems.
Gypsum is remarkable for its ability to push aside impurities as the crystals grow. The dry mud of the Rawl Series, OFD I, contained many clear selenite crystals when the passage was discovered. Little rosettes of gypsum also coat many dead straws in this part of the cave. Elsewhere, in other old dry parts of the cave, flakes of gypsum coat the walls.
A contrasting occurrence occurs in Agen Allwedd. The floor of Main Passage has many stalagmites. These are composed of gypsum. It has been shown that such forms are anti-stalactites, growing up from the base (Huff 1940). Sulphate bearing solutions rise through the mud floor, and evaporate at the surface, contributing to the underside of the stalagmites.
This article has tried to evaluate the contribution that chemistry, physics and biology can make towards our understanding of the formations that decorate our caves. Much remains to be learnt about the detailed structure of speleothems, and about the chemistry of their formation (see Hendy 1971). Long term studies of growth are essential to a better understanding of the 'pretties'that abound in our caves.
References:
Baker G, & Frostick A.C., 1947 J.Sed.Petrol. 17 pp 39-67
Broughton P.L., 1972 Secondary mineralization in the cavern environment. Stud. Speleol. 2 pp 191-207
Broughton P.L., 1977 Personal Communication
Franke H.W., 1961 Beitrage zur Morphologie des Höhlensinters III Höhle 12 pp 8-12
Franke H.W., 1962 Beitrage zur Morphologie des Höhlensinters IV Höhle 13 pp 77-82
Hendy, 1971 The isotopic geochemistry of speleothems. Geochimica et Cosmochimica Acta pp 801-824
Huff L.C., 1940 J. Geol. 48 pp 641-655
Mason-Williams M.A., 1961 Biological aspects of calcite deposition Memoria V della Rassegna Speleologica Italiana pp 1-4